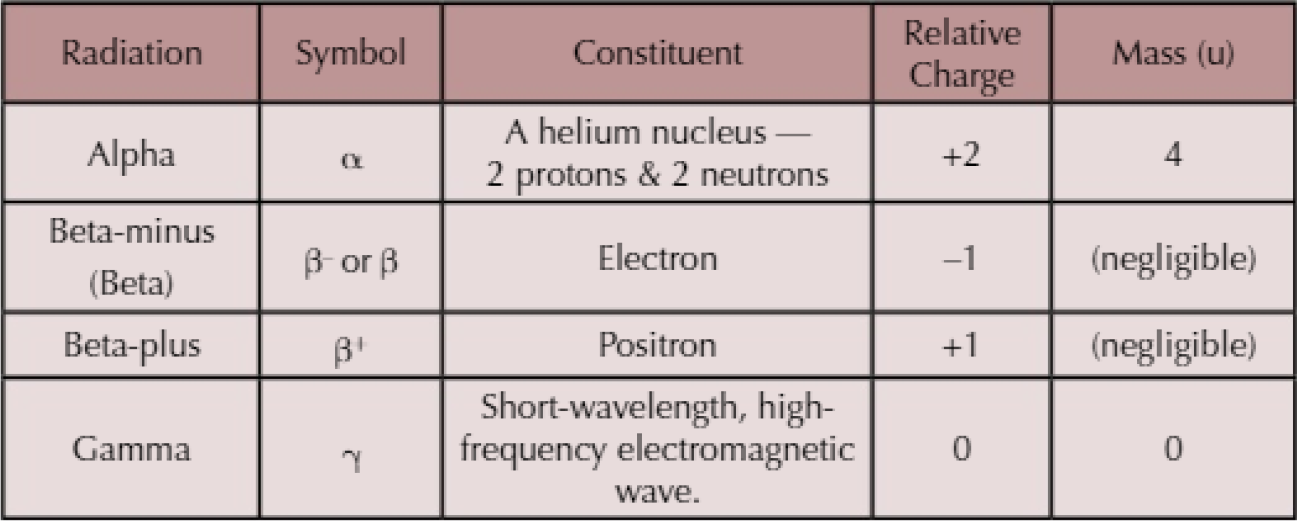
Properties of Nuclear Radiation
Types of Nuclear Radiation Diagram

Decay
- If an atomic nucleus is unstable, then it will break down to become more stable
- The nucleus decays by releasing energy and/or particles
- This happens until it reaches a stable form
- Decay is random and cannot be predicted.
Nuclear Equations: Alpha Decay
- \(_{91}^{231}Pa\to_{2}^4\alpha^{2+}+^{227}_{89}X\)
- \(^{223}_{87}Fr\to_{2}^4\alpha^{2+}+^{219}_{85}X\)
- \(^{149}_{62}Sm\to_{2}^4\alpha^{2+}+^{145}_{60}X\)
Alpha Radiation
- Alpha particles are strongly positive — so they can easily pull electrons off (ionise) atoms.
- Ionising an atom transfers some of the energy from the alpha particle to the atom.
- The alpha particle quickly ionises many atoms (about 1 0 000 ionisations per mm in air for each alpha particle) and loses all its energy.
- This makes alpha-sources suitable for use in smoke alarms because they allow current to flow, but won't travel very far.
- When smoke is present, the alpha particles can't reach the detector and this sets the alarm off.
- Although alpha particles can't penetrate your skin, sources of alpha particles are dangerous if they are ingested.
- They quickly ionise body tissue in a small area, causing lots of damage.
Beta Radiation
- The beta-minus particle has lower mass and charge than the alpha particle, but a higher speed. This means it can still knock electrons off atoms.
- Each beta particle will ionise about 100 atoms per mm in air, losing energy with each interaction. This lower number of interactions means that beta radiation causes much less damage to body tissue.
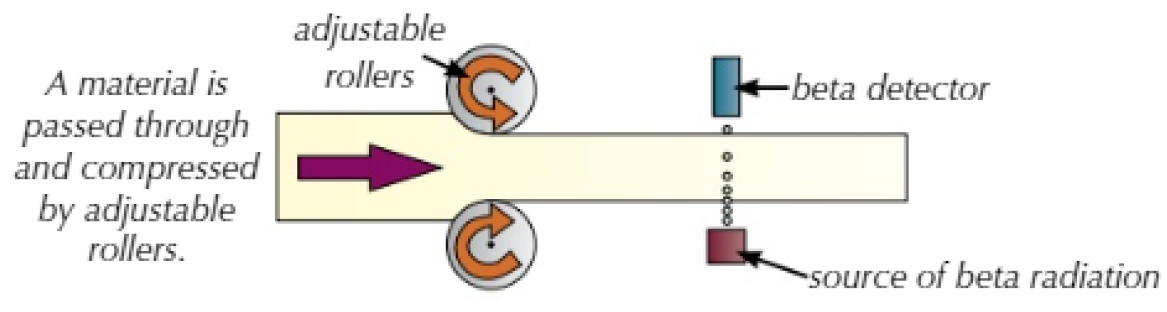
- When creating sheets of material, such as paper, aluminium foil or steel, beta radiation can be used to control its thickness.
- The material is flattened as it is fed through rollers.
- A radioactive source is placed on one side of the material, and a radioactive detector on the other. The thicker the material, the more radiation it absorbs and prevents from reaching the detector.
- If too much radiation is being absorbed, the rollers move closer together to make the material thinner.
- If too little radiation is being absorbed, they move further apart.
Positron B\(^+\) emission
- This emission is when a proton turns into a neutron and a postive anti-matter electron (positron) is created.
- These quickly annihalate when they interact with an electron
Gamma Radiation
- Gamma radiation is even more weakly ionising than beta radiation, so will do even less damage to body tissue.
- It is a wave and follows the inverse square law
- This means it can be used in medicine.
- Radioactive tracers are used to help diagnose patients without the need for surgery.
- A radioactive source with a short half-life to prevent prolonged radiation exposure is either eaten or injected into the patient.
- A detector, e.g. a PET scanner, is then used to detect the emitted gamma rays.
- Gamma rays can be used in the treatment of cancerous tumours — damaging cells and sometimes curing patients of cancer.
- Radiation damages all cells though — cancerous or not, and so sometimes a rotating beam of gamma rays is used.
- This lessens the damage done to surrounding tissue whilst giving a high dose of radiation to the tumour at the centre of rotation.
- Damage to other, healthy cells is not completely prevented however and treatment can cause patients to suffer side effects — such as tiredness and reddening or soreness of the skim
- Some forms of gamma ray treatments can also cause long term side effects like infertility.