E.M.F and Internal Resistance
Internal resistance
- Resistance comes from electrons colliding with atoms and losing energy.
- In a battery, chemical energy is used to make electrons move. As they move, they collide with atoms inside the battery — so batteries must have resistance.
- This is called internal resistance. Internal resistance is what makes batteries and cells warm up when they're used.
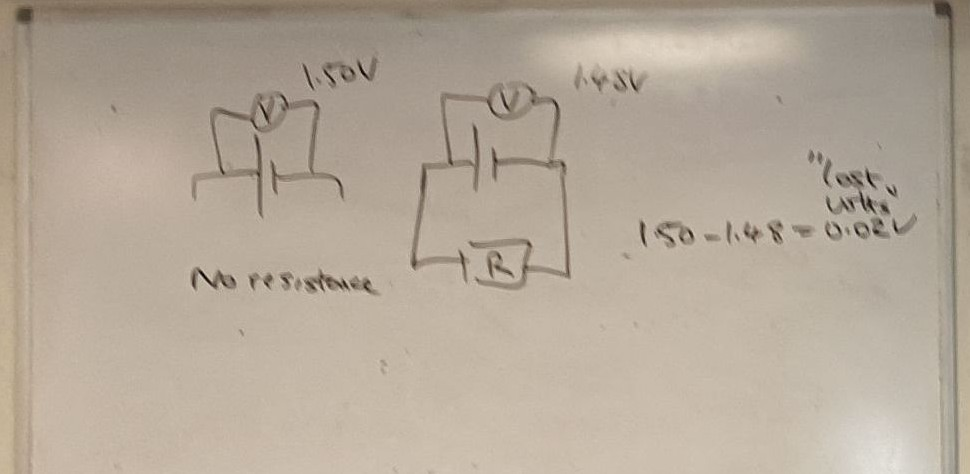
- \(\varepsilon=V+\text{lost volts}\)
- \(\varepsilon=V+Ir\) (ohms law)
- \(\varepsilon=Ir+IR\)
- \(\varepsilon=I(R+r)\)
- \(V=\varepsilon-Ir\)
EMF
- The amount of electrical energy the battery produces and transfers to each coulomb of charge is called its electromotive force or e.m.f. (e).
- Be careful, e.m.f. isn't actually a force. It's measured in volts.
- The potential difference (p.d.) across the load resistance (R) is the energy transferred when one coulomb of charge flows through the load resistance.
- This potential difference is called the terminal p.d. (V).
- If there was no internal resistance, the terminal p.d. would be the same as the e.m.f.
- However, in real power supplies, there's always some energy lost overcoming the internal resistance.
- The energy wasted per coulomb overcoming the internal resistance is called the lost volts (v).
Using a straight line equation for Internal Resistance
- \(\varepsilon=V+Ir\) can be rearranged into\(V=-rI+\varepsilon\).
- This is in the same format as \(y=mx+c\) which means we can use it in a straight line equation.
- \(V\) is on the y-axis, \(I/A\) is on the x-axis, \(r\) is the gradient, and \(\varepsilon\) is the y-intercept.