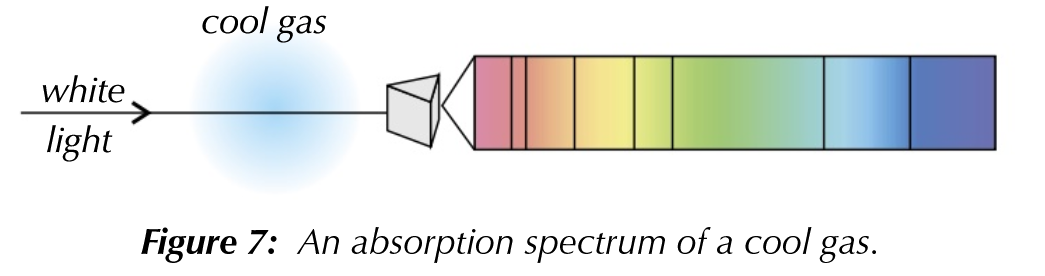
Line Absorption Spectra
Line Absorption Spectra
- The spectrum of white light is continuous, meaning if certain photon wavelengths are not present, then areas on the line spectrum will be dark.
- This happens when certain wavelengths of light are absorbed completely.
- This works when white light passes through a cool gas. At low temps, most electrons will be in their ground states. Photons of the correct wavelength will be absorbed to excite electrons to higher energy levels.