Discrete Energy Levels in Atoms
Discrete energy levels
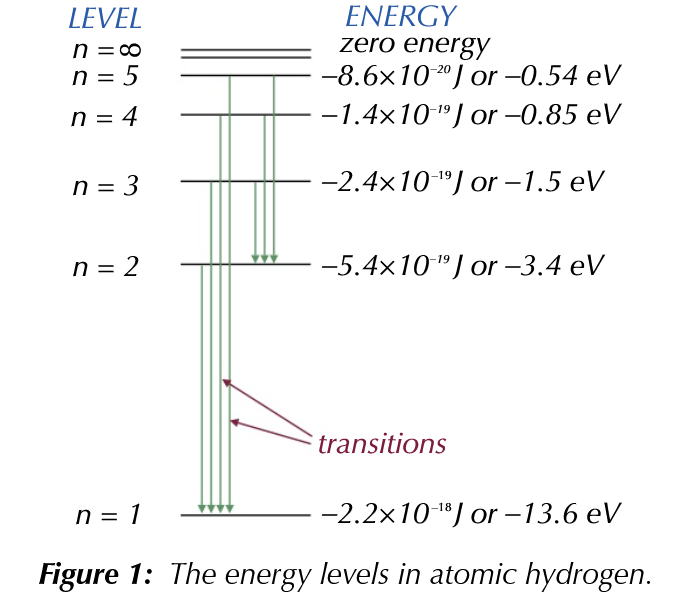
Summary
- Energy levels in atoms refer to the specific allowed energies that an electron in an atom can have.
- These energy levels are determined by the electric field created by the nucleus and other electrons in the atom, and are quantized, meaning that the allowed energy levels are discrete rather than continuous.
- The energy levels are typically represented by the letters n, where n can be any positive integer.
- The lowest energy level is known as the ground state, and higher energy levels are known as excited states.
- When an atom absorbs energy, an electron can be excited from the ground state to a higher energy level, and when it loses energy, an electron can return from an excited state to the ground state, emitting a photon of light in the process.