Electron Transitions
Calculate the wavelength of the photon released when an electron drops from energy level 3 to energy level 2
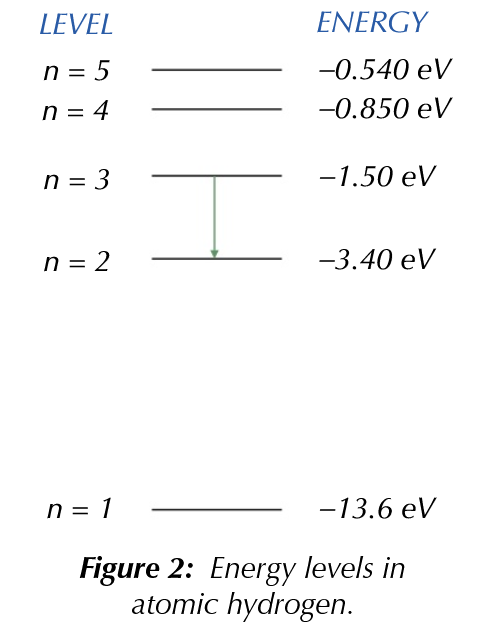
- \(3.4-1.5=1.9eV\)
- \(eV \to J\) = \(1.60\times 10^{-19}\)
- \(1.90\) \(eV\) \(\to\) \(3.04\times 10^{-19}J\)
- Use the equation \(E=\frac{hc}{\lambda}\)
- Rearrange to: \(\lambda=\frac{hc}{E}\)
- \(\frac{(6.63\times10^{-34})\times(3.00\times 10^8)}{3.04\times 10^{-19}}=6.54\times10^{-7}\)
Electron Transitions using Line Emission Spectrums
- When an electron jumps down an energy level, a photon is released.
- This photon has a wavelength that depends on how much energy is in it (how much energy was released from the electron) The equations
-
\[E=hf\]
-
\[E=\frac{hc}{\lambda}\]
-
\[\lambda=\frac{hc}{E}\]
-
- These equations can be used to calculate energy, wavelength and freqency.
Ionisation
- When an electron is removed from an atom, the atom is ionised.
- The energy of each energy level shows the amount of energy required to remove an electron from that particular energy level.
- The Ionisation energy shows how much energy is required to remove an electron from the ground state.